The Art and Science of Fireworks Displays
Art, Chemistry, Physics and Math Light Up the Fourth of July!
Article by: Hobart M. King, PhD, RPG

Colorful fireworks: The color of a fireworks burst is created by adding metallic compounds that flame with a known color to the stars within a fireworks shell. This photo shows fireworks bursts with a variety of color. Photographer was Kurume-Shimin. Photo used here under a Creative Commons license.

Aerial fireworks display: The vibrant colors in a fireworks display are made possible by minerals. Each color streak in the display above is formed by a burning particle as it travels through the air. Metal salts in the burning particles absorb heat energy and release it in the form of colored light. Image copyright iStockphoto / PapaBear.
Lights, Colors, Sounds, Shapes and Surprises!
Why do fireworks displays attract so many people? There are many answers to this question, but most people simply enjoy the bright explosions of light, color and sound. Others enjoy being surprised by the shape and color of the fireworks bursts. These shapes and colors do not happen by chance. They are deliberately produced by a careful combination of art, chemistry, physics and math!
How Fireworks Work
An aerial fireworks burst is produced by launching a fireworks shell high into the air, where an explosion occurs. This explosion propels brightly burning particles (known as "stars") in many directions. Each streak of light in the firework photos on this page is a burning "star" flying through the air.
Examine the diagram of a fireworks shell below and read the caption to better understand what happens in the air when this explosion occurs.

Fireworks launching equipment: Aerial fireworks shells are launched from short metal pipes known as "mortars." The wires running into the mortars carry a jolt of electricity that ignites an explosive charge in the bottom of the mortar. The explosion ignites the shell's fuse and launches it high into the air. Image copyright iStockphoto / garcia8914.
| Anatomy of an Aerial Fireworks Shell |
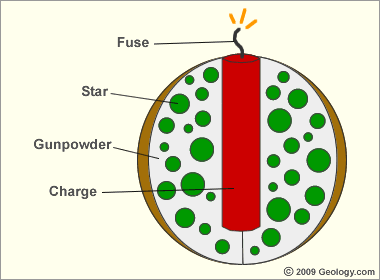
Anatomy of an Aerial Fireworks Shell: When a fireworks shell is launched into the air, the fuse is burning. The fuse is just the right length to ignite the explosive charge at the desired altitude. When the charge explodes it instantly ignites the gunpowder, increasing the size and force of the explosion. This explosion ignites the stars and throws them outwards in all directions. The stars burn as they travel through the air to produce the streaks of a brightly colored fireworks burst.

Fireworks in Germany: Colorful fireworks bursts from a celebration in Düsseldorf, Germany. Image copyright iStockphoto / Freder.
What Causes the Colors?
|
Chemistry holds the secrets to the color of a fireworks burst. The colors that you see in the sky are determined by metallic compounds that are deliberately added in very small amounts to the stars when they are manufactured.
As the stars burn, the metal atoms absorb energy, become excited and emit a specific color of light. Some of the metals that produce the colors of fireworks are tabulated here.
| Controlling the Appearance of the Burst |
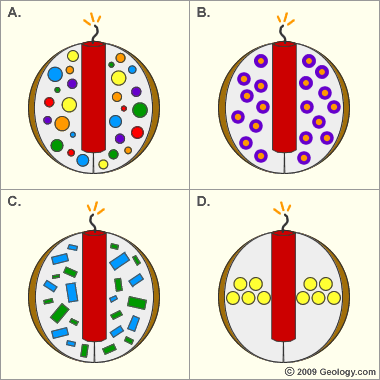
Colors and shapes of fireworks: A) Multicolor fireworks bursts can be produced by loading the charge with stars containing salts of various metals. Different sizes of stars will produce streaks of different lengths and brightness.
B) The outer part of a star ignites first and the inner part burns last. Stars that change colors as they fly have an outer layer that contains one metal salt and an inner core that contains another metal salt.
C) Changing the size and shape of the stars will produce bursts of different sizes, shapes and speeds.
D) Careful placement of the stars will change the shape of the burst. Rounds, rings, palms, willows, chrysanthemums and other burst shapes can be produced. An infinite number of bursts can be produced by carefully varying the shape, size, density, and composition of the stars.
The Mechanics of an Aerial Fireworks Burst
The people who make fireworks are really clever. They combine a knowledge of chemistry and physics with artistic ingenuity to produce an infinite variety of fireworks bursts. How do they do it? They change the size, shape, density, composition and placement of the stars within the fireworks shell. By doing this they change the shape, speed, direction, burn rate and color of the aerial burst.
They can also put shells within shells for multiple explosions and bursts. Or, they can include firecrackers, whistles or other noisemakers. Ingenious people can build fireworks shells for an infinite number of visual effects.
How Did They Do That?
The next time you go to a Fourth of July fireworks display, study the different types of bursts and imagine how they might have been accomplished.
You can probably imagine how many of them are done.
An ABC News video made to help people in California stay safe while using fireworks.
A fireworks safety video from the Consumer Product Safety Commission.

Fireworks in Indiana: Fireworks over Indianapolis, IN. Image copyright iStockphoto / Alexeys.

Fireworks in California: Fireworks over San Diego, CA. Image copyright iStockphoto / Njari.
Find Other Topics on Geology.com:

|

| ||

|

| ||

|

| ||

|

|









