Uses of Zinc
The metal that is key to preventing corrosion
Republished from United States Geological Survey Fact Sheet 2011-3016 by S.J. Kropschot and Jeff L. Doebrich

Zinc: Refined zinc metal is bluish-white when freshly cast; it is hard and brittle at most temperatures and has relatively low melting and boiling points. Image copyright iStockphoto / Svengine.
Historic Uses of Zinc
Centuries before it was identified as an element, zinc was used to make brass (an alloy of zinc and copper) and for medicinal purposes. Metallic zinc and zinc oxide were produced in India sometime between the 11th and 14th centuries and in China in the 17th century, although the discovery of pure metallic zinc is credited to the German chemist Andreas Marggraf, who isolated the element in 1746.
Sphalerite: The Primary Ore
Sphalerite (zinc sulfide) is the primary ore mineral from which most of the world's zinc is produced, but a number of other minerals that do not contain sulfide contain zinc as a major component. Much of the early zinc production was from nonsulfide deposits; however, as these resources were exhausted, production shifted to sulfide deposits. In the past 30 years, advances in extractive metallurgy have resulted in renewed interest in nonsulfide zinc deposits.

Zinc galvanizing: About one-half of the zinc that is produced is used in zinc galvanizing, which is the process of adding thin layers of zinc to iron or steel to prevent rusting. This photo shows the surface of a sheet of metal with a galvanized zinc coating. The different color domains across the sheet are caused by zinc crystals in different crystallographic orientations reflecting different amounts of light. Image copyright iStockphoto / Stephen Sweet.
Refined Zinc Metal
Refined zinc metal is bluish-white when freshly cast; it is hard and brittle at most temperatures and has relatively low melting and boiling points. Zinc alloys readily with other metals and is chemically active. On exposure to air, it develops a thin gray oxide film (patina), which inhibits deeper oxidation (corrosion) of the metal. The metal's resistance to corrosion is an important characteristic in its use.

Zinc alloys: The second-leading use of zinc is as an alloy; the zinc is combined with copper (to form brass) and with other metals to form materials that are used in automobiles, electrical components, and household fixtures. Brass fixture image copyright iStockphoto / Vova Kalina.
Uses of Zinc Today
Zinc is currently the fourth most widely consumed metal in the world after iron, aluminum, and copper. It has strong anticorrosive properties and bonds well with other metals. Consequently, about one-half of the zinc that is produced is used in zinc galvanizing, which is the process of adding thin layers of zinc to iron or steel to prevent rusting.
The next leading use of zinc is as an alloy; the zinc is combined with copper (to form brass) and with other metals to form materials that are used in automobiles, electrical components, and household fixtures. A third significant use of zinc is in the production of zinc oxide (the most important zinc chemical by production volume), which is used in rubber manufacturing and as a protective skin ointment.
Zinc is also important for health. It is a necessary element for the proper growth and development of humans, animals, and plants. The adult human body contains between 2 and 3 grams of zinc, which is the amount needed for the body's enzymes and immune system to function properly. It is also important for taste, smell, and to heal wounds. Trace amounts of zinc occur in many foods, such as oysters, beef, and peanuts.

Zinc oxide: The third significant use of zinc is in the production of zinc oxide (the most important zinc chemical by production volume), which is used in rubber manufacturing and as a protective skin ointment. Zinc oxide image copyright iStockphoto / Demiren.
Where Does Zinc Come From?
Research to better understand the geologic processes that form mineral deposits, including those of zinc, is an important component of the USGS Mineral Resources Program. Zinc is commonly found in mineral deposits along with other base metals, such as copper and lead. Zinc deposits are broadly classified on the basis of how they are formed. Zinc is produced mainly from three types of deposits: sedimentary exhalative (Sedex), Mississippi Valley type (MVT), and volcanogenic massive sulfide (VMS).
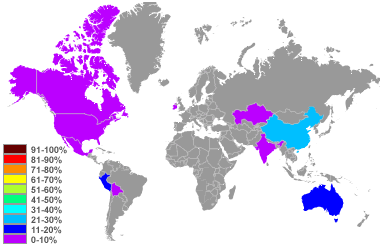
Zinc production map: World top zinc producers in percent of global supply produced in 2010. Image based upon data in the United States Geological Survey Mineral Commodity Summary, January 2011.
Sedimentary Exhalative Deposits
Sedex deposits account for more than 50 percent of the world's zinc resources and are formed when metal-rich hydrothermal fluids are released into a water-filled basin (usually an ocean), resulting in the precipitation of ore-bearing material within basin-floor sediments. The world's largest zinc mine, the Red Dog mine in Alaska, is developed in a Sedex deposit.
Mississippi Valley Type Deposits
MVT deposits are found throughout the world and get their name from deposits that occur in the Mississippi Valley region of the United States. The deposits are characterized by ore mineral replacement of the carbonate host rock; they are often confined to a single stratigraphic layer and extend over hundreds of square kilometers. MVT deposits were a major source of zinc in the United States from the 19th century through the mid-20th century.
Volcanogenic Massive Sulfide Deposits
In contrast to Sedex and MVT deposits, VMS deposits have a clear association with submarine volcanic processes. They also can contain significant amounts of copper, gold, and silver in addition to zinc and lead. The "black smoker" sea vents discovered during deep ocean expeditions are examples of VMS deposits being formed on the sea floor today.
Zinc Production and Reserves | ||
| Country | Production | Reserves |
| United States | 720 | 12,000 |
| Australia | 1,450 | 53,000 |
| Bolivia | 430 | 6,000 |
| Canada | 670 | 6,000 |
| China | 3,500 | 42,000 |
| India | 750 | 11,000 |
| Ireland | 350 | 2,000 |
| Kazakhstan | 480 | 16,000 |
| Mexico | 550 | 15,000 |
| Peru | 1,520 | 23,000 |
| Other Countries | 1,580 | 62,000 |
| Total (rounded) | 12,000 | 250,000 |
| Data is in thousand metric tons. Data from USGS Mineral Commodity Summary, January 2011. | ||
Worldwide Supply of and Demand for Zinc
In 2009, zinc was mined in six different States; however, the United States imported 76 percent of the refined zinc used domestically, primarily from Canada, Mexico, Kazakhstan, and the Republic of Korea, in descending order. Worldwide zinc consumption remained steady in 2008, as increased consumption in countries with emerging markets (such as China, Brazil, and India) offset declining consumption in Europe and the United States, according to International Lead and Zinc Study Group statistics.
Although many elements can be used as a substitute for zinc in chemical, electronic, and pigment applications, the demand for zinc galvanized products remains strong, especially in regions where significant infrastructure projects are being developed. The dramatic increase in the world's production (supply) and consumption (demand) of zinc in the past 35 years reflects demand in the transportation and communications sectors for such things as automobile bodies, highway barriers, and galvanized iron structures.
Ensuring Adequate Supplies of Zinc for the Future
To help predict where future zinc supplies might be located, USGS scientists study how and where identified zinc resources are concentrated in the Earth's crust and use that knowledge to assess the likelihood that undiscovered zinc resources exist. Techniques to assess mineral resource potential have been developed and refined by the USGS to support the stewardship of Federal lands and to better evaluate mineral resource availability in a global context.
In the 1990s, the USGS conducted an assessment of U.S. zinc resources and concluded that twice as much zinc remained to be found as had already been discovered. Specifically, the USGS found that less than 100 million metric tons of zinc had been discovered in the United States and estimated that about 210 million metric tons of zinc remained undiscovered.
Mineral resource assessments are dynamic. Because they provide a snapshot at a particular time and level of knowledge, the assessments must be updated as better data become available and new concepts are developed. For example, during reconnaissance geologic investigations in the late 1960s, USGS geologists noted the presence of widespread iron-oxide staining in the drainages of the western Brooks Range, Alaska.
The Red Dog Lead-Zinc Deposit
Followup studies led to the discovery of the Red Dog lead-zinc deposit. In late 1990, after 10 years of exploration and development work, the Red Dog mine in Alaska went into production and has since contributed greatly to the global zinc supply. Subsequent investigations of the area have resulted in a better understanding of the complex factors that controlled the formation of Red Dog and other deposits and provide the basis for assessment of similar deposits in similar geologic environments elsewhere. Other current research by the USGS involves updating mineral deposit models and mineral environmental models for zinc and other important nonfuel commodities and improving the techniques used to assess for concealed mineral resource potential. The results of this research will provide new information and decrease the amount of uncertainty in future mineral resource assessments.
Find Other Topics on Geology.com:

|

| ||

|

| ||

|

| ||

|

|









