
Uses of silver: Historically, silver has been used in coins, silverware, and jewelry, but today, these uses account for less than half of all silver consumption. Silver has become a material of innovation that appears in many unexpected places. Images copyright iStockphoto / Jorge Farres Sanchez, Tatiana Buzuleac, Nigel Spooner, and Stephanie Frey.
The White Metal
Silver, the white metal, has an illustrious reputation for its use in jewelry and coins, but today, silver's primary use is industrial. Whether in cell phones or solar panels, new innovations are constantly emerging to take advantage of silver's unique properties.
Silver is a precious metal because it is rare and valuable, and it is a noble metal because it resists corrosion and oxidation, though not as well as gold. Because it is the best thermal and electrical conductor of all the metals, silver is ideal for electrical applications. Its antimicrobial, non-toxic qualities make it useful in medicine and consumer products. Its high luster and reflectivity make it perfect for jewelry, silverware, and mirrors. Its malleability, which allows it to be flattened into sheets, and ductility, which allows it to be drawn into thin, flexible wire, make it the best choice for numerous industrial applications. Meanwhile, its photosensitivity has given it a place in film photography.
Because it is more abundant, silver is much less expensive than gold. Silver can be ground into powder, turned into paste, shaved into flakes, converted into a salt, alloyed with other metals, flattened into printable sheets, drawn into wires, suspended as a colloid, or even employed as a catalyst. These qualities ensure that silver will continue to shine in the industrial arena, while its long history in coinage and jewelry will sustain its status as a symbol of wealth and prestige.

Silver paste in electronics: Printed electronics like RFID tags rely on silver paste. Image copyright iStockphoto / JacobH.
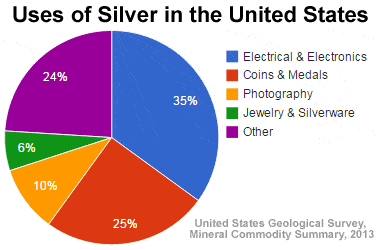
Uses of silver in the U.S.: The United States Geological Survey has tabulated the amount of silver used in the United States by category of use. This data is shown in the graphic above. The "Other" category accounts for almost a quarter of the silver used and is fragmented into hundreds of different uses. Many of these are described below.
Uses of Silver in Electronics
The number one use of silver in industry is in electronics. Silver's unsurpassed thermal and electrical conductivity among metals means it cannot easily be replaced by less expensive materials.
For example, small quantities of silver are used as contacts in electrical switches: join the contacts, and the switch is on; separate them and the switch is off. Whether turning on a bedroom light using a conventional switch or turning on a microwave using a membrane switch, the result is the same: the current can pass through only when the contacts are joined. Automobiles are full of contacts that control electronic features, and so are consumer appliances. Industrial strength switches use silver, too.
How does silver get from the earth to these electronic devices? Silver comes from silver mines or from lead and zinc mines from which silver is a by-product. Smelting and refining removes silver from the ore. Then, the silver is usually shaped into bars or grains. Electronics demand silver of the highest purity: 99.99% pure, also known as having a fineness of 999.9.
Dissolving pure silver in nitric acid produces silver nitrate, which can be formed into powder or flakes. This material, in turn, can be fabricated into contacts or silver pastes, like conductive paste made with a silver-palladium alloy.
Silver paste has many uses, such as the membrane switch already mentioned and the rear defrost in many cars. In electronics, circuit paths, as well as passive components called multilayer ceramic capacitors (MLCCs), rely on silver paste. One of the fastest growing uses of silver paste is in photovoltaic cells for the production of solar energy.
Nanosilver, silver with an extremely small particle size (1-100 nanometers, that is 1-100 billionths of a meter), provides a new frontier for technological innovation, requiring much smaller amounts of silver to get the job done. Printed electronics work by using nanosilver conductive inks. One example of a printed electronic is the electrode in a supercapacitor, which can charge and discharge repeatedly and quickly. Regenerative breaking is an automotive innovation that allows the kinetic energy of a slowing vehicle to be stored in a supercapacitor for reuse. Radio frequency identification (RFID) tags offer another powerful application of printed electronics. These tags are better than bar codes for tracking inventory because they store more information and can be read from a greater distance, even without a direct line of sight.
Silver has its place in consumer electronics, too. Your plasma television set may rely on silver for more than just the on-off switch if it contains a silver electrode aimed at giving a higher quality image. Light emitting diodes (LED) also use silver electrodes to produce low-level, energy efficient light. Meanwhile the DVDs and CDs you play probably have a thin silver recording layer.
Another electronic application of silver is in batteries that employ silver oxide or silver zinc alloys. These light-weight, high-capacity batteries perform better at high temperature than other batteries. Silver-oxide is used in button batteries that power cameras and watches, as well as in aerospace and defense applications. Silver-zinc batteries offer an alternative to lithium batteries for laptop computers and electric cars.
On the cutting edge of technology are superconductors. Silver is not a superconductor, but when paired with one, the two together can transmit electricity even faster than the superconductor alone. At very low temperatures, superconductors carry electricity with little or no electrical resistance. They can be used to generate magnetic energy for turning motors or propelling magnetic levitation trains.
The myriad applications of silver in electronics offer an eye-opening view into how one of the most famous metals in history has become a cutting edge material of the future. Due in part to its unique property of having the highest thermal and electrical conductivity of all metals, silver is often a must-have over other, less expensive materials.

Silver in solar panels: Silver paste contacts form bus bars and grid lines that draw electrical current from the semiconducting surface of a photovoltaic cell. Image copyright iStockphoto / Gyuszko.
Uses of Silver in Energy
As mentioned previously, silver paste is used to make solar panels. Silver paste contacts printed onto photovoltaic cells capture and carry electrical current. This current is produced when energy from the sun impacts the semiconducting layer of the cell. Photovoltaic cells are one of the fastest growing uses of silver.
Silver's reflectivity gives it another role in solar energy. It reflects solar energy into collectors that use salts to generate electricity.
Nuclear energy also uses silver. The white metal is often employed in control rods to capture neutrons and slow the rate of fission in nuclear reactors. Inserting the control rods into the nuclear core slows the reaction, while removing them speeds it up.

Silver brazing and soldering: Silver brazing and soldering ensures a tight joint between metal pipes. Image copyright iStockphoto / bypicart.
Uses of Silver in Brazing and Soldering
Brazing and soldering make use of silver's high tensile strength and ductility to create joints between two metal pieces. Brazing takes place at temperatures above 600°C, while soldering takes place at temperatures below 600°C. Silver scrap can be used in brazing and soldering because these processes do not require very pure silver. Brazing and soldering produce tight joints for everything from heating and air conditioning vents to plumbing. Silver's antibacterial properties and non-toxicity to humans make it a great replacement for lead-based bonds between water pipes.

Silver wire: Silver wire can be woven into a metal mesh and used as a catalyst. Granular silver also makes a good catalyst. Image copyright iStockphoto / Greg801.
Uses of Silver in Chemical Production
Silver acts as a catalyst to produce two important chemicals: ethylene oxide and formaldehyde. Ethylene oxide is used to produce molded plastics, such as plastic handles, and flexible plastics, such as polyester. It is also a major ingredient in antifreeze. Formaldehyde is used to make solid plastics and resins and as a protective coating. It is also used as a disinfectant and embalming agent. As a catalyst, silver increases the speed of reactions without getting used up.

Silver coins and bullion: Silver coins have been minted for thousands of years. Silver bullion is still a popular investment choice today. Image copyright iStockphoto / shakzu.
Uses of Silver in Coins and Investments
Silver has traditionally served, with gold, as the metal used in coins. As a precious metal, silver is rare and valuable, making it a convenient store of wealth. In the past, people accumulated their wealth in the form of silver coins; today, they invest in investment-grade silver bullion. The fact that silver does not corrode and only melts at a relatively high temperature, means that it can last, and the fact that it has high luster makes it attractive. Its malleability makes silver a good choice for designing and minting local currency.
In greater abundance, and therefore less expensive, than gold, silver has been used more prevalently as currency. Silver was mined and used in trading several thousands of years BC and was first minted into silver coins in the Mediterranean region many hundreds of years BC. Until the 20th century, many countries used a silver or gold standard, backing up the value of currency with the presence of gold or silver in the treasury. Today, countries use less expensive metals, such as copper and nickel, to produce coins, and they use fiat currency, in which government regulation controls the value, instead of a gold or silver standard.
Still, silver retains its value as a commodity. Many individuals choose to invest in silver through financial instruments, like stocks and mutual funds, or by actually buying and storing 99.9% pure silver bullion bars, coins, or medallions. Countries sometime produce silver collector's edition coins, which they sell to buyers at a price exceeding the value of the silver used to make the coin.

Silver rings: Silver, precious and lustrous, makes beautiful, long-lasting jewelry. Image copyright iStockphoto / pixeljuice.
Uses of Silver in Jewelry and Silverware
Jewelry and silverware are two other traditional uses of silver. Malleability, reflectivity, and luster make silver a beautiful choice. Because it is so soft, silver must be alloyed with base metals, like copper, as in the case of sterling silver (92.5% silver, 7.5% copper). Even though it resists oxidation and corrosion, silver can tarnish, but with a little polish, it can shine for a lifetime. Because it is less expensive than gold, silver is a popular choice for jewelry and a standard for fine dining. Silver-plated base metals offer a less costly alternative to silver. Silver dishes and plates may accompany silverware, and these can often be ornately crafted works of art. For example, Paul Revere (1734-1818), known to most for his midnight ride at the start of the American Revolution, was a silversmith by trade, and some of his artwork is still on display at the Boston Museum of Fine Arts.

Silver in photography film: Silver halide in photographic film responds to light, leaving a latent image behind. Image copyright iStockphoto / Njari.
Uses of Silver in Photography
Photography had been one of the primary industrial uses of silver until the recent rise of digital media. Traditional film photography relies on the light sensitivity of silver halide crystals present in film. When the film is exposed to light, the silver halide crystals change to record a latent image that can be developed into a photograph. The accuracy of this process makes it useful for non-digital consumer photography, film, and X-rays.
The silver used in film photography should not be confused with the "silver screen" of cinema. This phrase refers not to the silver in the film itself, but to the silver lenticular screen onto which early films were projected.

Silver is used in ointments: Some ointments take advantage of silver's antibacterial qualities to protect wounds against infection. Image copyright iStockphoto / cglade.
Uses of Silver in Medicine
Silver ions act as a catalyst by absorbing oxygen, which kills bacteria by interfering with their respiration. This antibiotic property, along with its non-toxicity, has given silver an essential role in medicine for thousands of years. Before widespread use of antibiotics, silver foil was wrapped around wounds to help them heal, and colloidal silver and silver-protein complexes were ingested or applied topically to fight illness. Silver has also been used in eye drops and in dental hygiene to cure and prevent infection.
While silver is not toxic, repeat intake of small amounts of silver over time can result in argyria. In people with this condition, silver builds up in body tissue, giving it a gray-blue appearance when exposed to the sun. In addition, the ingestion of large amounts of silver can have negative effects on the body. For these reasons, medical doctors discourage the use of colloidal silver, discounting claims by some that colloidal silver is a cure-all dietary supplement.
Today, the presence of antibiotic-resistant superbugs increases the demand for silver in hospitals. Small amounts of silver can coat hospital surfaces and medical equipment to prevent the spread of pathogens. Silver in surgical equipment, wound dressings, and ointments protects wounds from infection. Silver sulfadiazine is especially useful for burn victims because it kills bacteria while also allowing the skin to regrow. Silver ion treatments can heal bone infections and allow regeneration of damaged tissue.

Silver in windows of skyscrapers: Many modern skyscrapers have window glass that is coated with silver to reflect sunlight and reduce air conditioning costs. Image copyright iStockphoto / sankai.
Uses of Silver in Mirrors and Glass
Silver is almost completely reflective when polished. Since the 19th century, mirrors have been made by coating a transparent glass surface with a thin layer of silver, though modern mirrors also use other metals like aluminum. Many windows of modern buildings are coated with a transparent layer of silver that reflects sunlight, keeping the interior cool in the summer. In aerospace, silver-coated tiles protect spacecraft from the sun.

Silver-coated ball bearings: Silver-coated ball bearings reduce friction in engines. Image copyright iStockphoto / felixR.
Uses of Silver in Engines
Engine bearings rely on silver. The strongest bearing is made from steel that has been electroplated with silver. Silver's high melting point allows it to withstand the high temperature of engines. Silver also acts like a lubricant to reduce friction between a ball bearing and its housing. Due to its ability to absorb oxygen, silver is being researched as a possible substitute for platinum to catalyze oxidation of matter collected in diesel engine filters.

Silver awards: Silver often signifies second place and is used in medals and other awards. Image copyright iStockphoto / Vonkara1.
Uses of Silver in Awards
Due to its status as a precious metal, ranked second only to gold, silver is often used to award second place. The most famous silver award is the second-place Olympic Silver Medal. Silver also symbolizes honor, valor, and accomplishment, which is why many military organizations, employers, clubs, and associations use silver or silver-colored awards to honor individuals for their contributions.

Silver in water filters: A silver coating prevents bacterial build-up in carbon-based water filtration systems. Image copyright iStockphoto / RaginaQ.
Uses of Silver for Water, Food, Hygiene
Silver's antibacterial properties have been applied for thousands of years, long before the discovery of microbial organisms, because silver containers and coins were known to prevent spoilage of liquids. Today, a silver coating prevents bacterial build-up in carbon-based water filters, while silver ions in water purification systems carry oxygen that oxidizes and kills microbes. Silver-copper ions can even replace corrosive chlorine to sanitize pools and tanks.
The antimicrobial properties of silver that make it useful to medicine and water purification are now being applied in food and hygiene. Nanosilver coatings are applied to food packages and refrigerators. And many new consumer products, such as washing machines, clothing, and personal hygiene products tout the benefits of antibacterial silver.

Did You Know? Most of the world's silver is produced as a by-product of lead mining. The mineral galena (shown above) is a combination of lead and sulfur. However, a small amount of silver usually substitutes for lead in the mineral's crystal structure. At many galena mines, enough silver can be present in the galena that the value of the silver greatly exceeds the value of the lead that is mined. The financial reality of this is that the supply of silver is more dependent upon the amount of lead that is being mined than the price of silver. Specimen and photo by Arkenstone / www.iRocks.com.
| Silver Information |
|
[1] Silver: The Indispensable Metal: The Silver Institute, www.silverinstitute.org.
[2] Silver: Mineral Commodity Summaries: United States Geological Survey, www.usgs.gov. [3] Silver: Minerals Yearbook: United States Geological Survey, www.usgs.gov. |
Other Uses of Silver
Other traditional uses of silver exist. For example, silver is one ingredient in the amalgam used to fill dental cavities, though this approach has been largely replaced by other materials due to the presence of toxic mercury in the amalgam. Silver has also been used to plate instruments, such as flutes.
Today, silver is being applied to many new uses. Silver is one of many options for replacing toxic chromated copper arsenate as a wood preservative. Nanosilver inks and coatings on paper tout their ability to prevent the spread of bacterial infection. Silver metal glass, produced by cooling silver quickly, offers durable strength that resists deformation. Silver-based ionic liquids, which are in a liquid state at room temperature, can be used to clean up petroleum waste products. Silver in fabric allows touch screen users to keep their gloves on during cold weather.
Silver seems to have as many uses as the human imagination can develop. Traditional works of silver, like jewelry and silverware, rely on the creativity of artists. Modern uses depend on the creative exploits of scientists and engineers to meet the changing demands of consumers and industries. While some uses rise and fall, such as the use of silver in photographic film, other uses may continue to grow, such as the burgeoning production of photovoltaic cells for solar energy. Silver's unique properties, especially its high thermal and electrical conductivity, its reflectivity, and its antibacterial qualities, make it difficult to replace, like a one-of-a-kind silver ring.
Find Other Topics on Geology.com:

|

| ||

|

| ||

|

| ||

|

|









