Galena
The primary ore of lead that is sometimes mined for its silver content
Article by: Hobart M. King, PhD

Galena: Photograph of a nice cubic galena crystal with adjacent calcite crystals. The galena crystal is about two inches on a side. Collected from the Sweetwater Mine, Reynolds County, Missouri. Specimen and photo by Arkenstone / www.iRocks.com.
What is Galena?
Galena is a lead sulfide mineral with a chemical composition of PbS. It is the world's primary ore of lead and is mined from a large number of deposits in many countries. It is found in igneous and metamorphic rocks in medium- to low-temperature hydrothermal veins. In sedimentary rocks it occurs as veins, breccia cements, isolated grains, and as replacements of limestone and dolostone.
Galena is very easy to identify. Freshly broken pieces exhibit perfect cleavage in three directions that intersect at 90 degrees. It has a distinct silver color and a bright metallic luster. Galena tarnishes to a dull gray. Because lead is a primary element in galena, the mineral has a high specific gravity (7.4 to 7.6) that is immediately noticed when picking up even small pieces. Galena is soft with a Mohs hardness of 2.5+ and produces a gray to black streak. Crystals are common and they usually are cubes, octahedrons, or modifications.
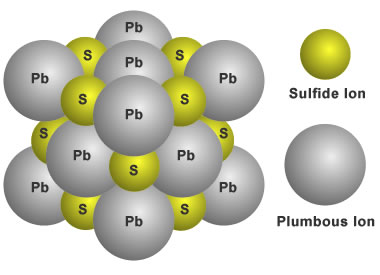
Structure of galena: Galena has a chemical composition of PbS. That means it contains an equal number of lead and sulfide ions. The ions are arranged in a cubic pattern that repeats in all directions. This structure is what causes crystals of galena to have a cubic form and causes galena to break in three directions at right angles.
Physical Properties of Galena |
|
| Chemical Classification | Sulfide |
| Color | Fresh surfaces are bright silver in color with a bright metallic luster, tarnishes to a dull lead gray |
| Streak | Lead gray to black |
| Luster | Metallic on fresh surfaces, tarnishes dull |
| Diaphaneity | Opaque |
| Cleavage | Perfect, cubic, three directions at right angles |
| Mohs Hardness | 2.5+ |
| Specific Gravity | 7.4 to 7.6 |
| Diagnostic Properties | Color, luster, specific gravity, streak, cleavage, cubic or octahedral crystals. |
| Chemical Composition | Lead sulfide, PbS |
| Crystal System | Isometric |
| Uses | An ore of lead |

Argentiferous galena: Argentiferous galena from Coeur d'Alene, Idaho. Specimen is approximately 2-1/2 inches (6.4 centimeters) across. Argentiferous galena has a silver content that is often high enough for the galena to be mined as an ore of silver. Some galena mines receive more revenue from their silver than from their lead production.
Argentiferous Galena - The Silver Ore
The typical specimen of galena is about 86.6% lead and 13.4% sulfur by weight. However, some specimens of galena contain up to a few percent silver by weight. They are called "argentiferous galena" because of their silver content. In these specimens, silver can substitute for lead in the atomic structure of the galena, or it can occur in tiny grains of silver minerals included in the galena.
Silver within the galena disrupts the crystal structure, which often causes the galena to have curved cleavage faces. This tiny bit of knowledge can be a powerful prospecting tool. In addition to silver, galena can contain minor amounts of antimony, arsenic, bismuth, cadmium, copper, and zinc. Sometimes selenium substitutes for sulfur in galena.

Cleavage fragments of galena: One of the most diagnostic properties of galena is its ability to break by cleavage in three directions that intersect at right angles. This forms cleavage fragments that are cubic and rectangular in shape. This photo shows pieces of crushed galena that clearly exhibit the right angle cleavage. This characteristic cleavage is caused by the mineral's cubic internal structure as shown above. Photo copyright iStockphoto / Tyler Boyes.
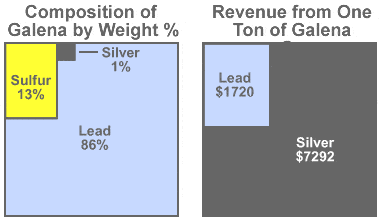
Galena value: Some mines produce more revenue from the silver content of their galena than from the lead content. Assume that we have a mine that produces argentiferous galena with an average composition of 86% lead, 13% sulfur and just 1% silver (as shown in the diagram on the left).
If the silver price is $25 per troy ounce and the lead price is $1 per avoirdupois pound, the value of the lead in one ton of ore will be $1720, while the value of the silver in that same ton of ore will be $7292 (as shown in the diagram on the right).
The small amount of silver has a huge impact on revenue because at the prices assumed, silver is 364 times more valuable than an equal weight of lead. It is easy to understand why mining companies get excited by argentiferous galena! Even though galena is the ore being removed and lead makes up the bulk of the product, these mines are often called "silver mines."

Smelting metals: Galena is one of the easiest ores to smelt. It can simply be placed in a fire and then lead can be recovered from under the ashes when the fire goes out. Archaeologists have found evidence that lead was smelted as early as 6500 BC in what is now Turkey [1]. Small amounts of silver were refined from lead by the Romans about 2000 years ago [2]. United States public domain image by Georgius Agricola.
Smelting Galena
Galena is very easy to smelt. If rocks that contain galena are placed in a fire, lead can be collected from below the ashes after the fire burns out. People have taken advantage of this simple smelting for thousands of years. Archaeologists have found lead beads and statues in Turkey that date back to about 6500 BC [1]. Lead is probably the first metal to have been processed from an ore. The ancient Romans made lead pipe and used it as indoor plumbing. (Plumbum is the Latin word for lead. The word "plumbing" and our use of "Pb" as the chemical symbol for lead come from the ancient Romans.)
The ancient Greeks and Romans were able to separate silver from lead about 2000 years ago [2]. Many of the Roman lead ingots were inscribed "Ex Arg" or "Ex Argent" to signify that the silver had been removed from the lead. The Greeks were able to desilver lead to a 0.02 percent silver content and the Romans to a 0.01 percent silver content [3]. It is surprising that they were able to realize that the lead contained silver and amazing that they were able to develop such an efficient method of refining!
Alteration of Galena
Galena weathers easily. Fresh surfaces of galena tarnish rapidly from a silver metallic luster to a dull gray to dull black color. When exposed to the elements or buried in soil, galena quickly weathers to anglesite, cerussite, pyromorphite, or another lead mineral. These minerals are often used in prospecting. When they are found at the surface, they often reveal that galena is present below.

The best way to learn about minerals is to study with a collection of small specimens that you can handle, examine, and observe their properties. Inexpensive mineral collections are available in the Geology.com Store. Image copyright iStockphoto / Anna Usova.
Does It Really "Snow" Galena on Venus?
The planet Venus has an inhospitable environment where volcanoes vent superheated gases into the atmosphere. Sulfur and lead are among the gases erupted from the volcanoes on Venus. They remain in the gaseous phase until they are high enough in the atmosphere to condense.
In 2004, researchers at Washington University in St. Louis provided plausible evidence that "heavy metal snow" - which is most likely a combination of lead sulfide (galena) and bismuth sulfide - falls on the higher elevations of Venus [4].

Galena crystal radio: One of the most interesting uses of galena was in early crystal radios. The operation of these radios required alternating current to be converted into a pulsing direct current. For that to occur, a semiconductor material was used to limit the flow of electricity to one direction. The alternating current flowed through a wire, known as a cat's whisker, into a semiconductor crystal, which was usually a crystal of galena, which only allowed flow in a single direction. Image copyright iStockphoto / Greg_H.
Uses of Galena
Galena is a very important mineral because it serves as an ore for most of the world's lead production. It is also a significant ore of silver. Galena has very few uses beyond its service as an ore, but that should not diminish its importance to society.
The number one use of lead today is in the lead-acid batteries that are used to start automobiles. The typical auto battery contains about twenty pounds of lead and must be replaced every four or five years. There are billions of these batteries in the United States alone. Lead-acid batteries are also used as standby power supplies for computer networks, communication facilities, and other critical systems. Lead is also one of the metals used in energy storage systems associated with power generation and hybrid vehicles.
| Galena Information |
|
[1] Lead Fact Sheet: General Information and History, Stanford University, General Health and Safety Program, last accessed August 2022.
[2] On the Nature of Metals (De Re Metallica): Georgius Agricola, 1556. Translated by Herbert Clark Hoover and Lou Henry Hoover, republished by Project Gutenberg. [3] Pliny the Elder on Science and Technology: John F. Healy, Oxford University Press, page 324, 1999. [4] 'Heavy Metal' Snow on Venus is Lead Sulfide: by Carolyn Jones Otten, press release of Washington University in St. Louis, February 2004. |
Lead Safety
Many uses of lead and lead compounds have been discontinued or significantly reduced over the past few decades in response to health concerns. Some of these uses include lead in residential paints, motor vehicle fuels, solder, ammunition, fishing weights, ceramic glazes, pesticides, cosmetics, glass, plastics, alloys and many other products. For this reason, many schools have removed galena from student mineral kits and have replaced it with a mineral with a lower level of concern.
| More Minerals |
 |
Herkimer Diamonds |
 |
The Acid Test |
 |
Tumbled Stones |
 |
Zircon |
 |
Fool*s Gold |
 |
Kyanite |
 |
Rock Tumblers |
 |
Rhodochrosite |

Find Other Topics on Geology.com:

|

| ||

|

| ||

|

| ||

|

|
