Home » Oil and Gas » Methane Hydrate
Methane Hydrate
The world's largest natural gas resource is trapped beneath permafrost and ocean sediments.
Article by: Hobart M. King, PhD, RPG
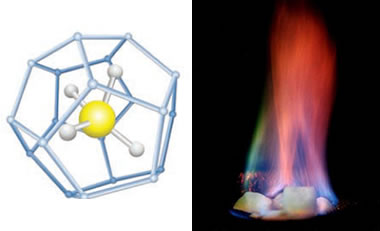
Methane hydrate: On the left is a ball-and-stick model of methane hydrate showing the central methane molecule surrounded by a "cage" of water molecules. Other hydrocarbon molecules such as pentane and ethane can occupy the central position in this structure. (United States Department of Energy image). On the right is a burning specimen of methane hydrate ice (United States Geological Survey image).

Methane hydrate "cement" in conglomerate?: This photo shows a core sample of the methane hydrate zone in the Mallik Test Well. This well penetrates permafrost deposits in Canada's Mackenzie River Delta area. This portion of the core shows gravels cemented into a "conglomerate" by methane hydrate ice. Click to enlarge image.
The Next Energy "Game Changer"?
As natural gas from shale becomes a global energy "game changer," oil and gas researchers are working to develop new technologies to produce natural gas from methane hydrate deposits. This research is important because methane hydrate deposits are believed to be a larger hydrocarbon resource than all of the world's oil, natural gas and coal resources combined. [1] If these deposits can be efficiently and economically developed, methane hydrate could become the next energy game changer.
Enormous amounts of methane hydrate have been found beneath Arctic permafrost, beneath Antarctic ice, and in sedimentary deposits along continental margins worldwide. In some parts of the world they are much closer to high-population areas than any natural gas field. These nearby deposits might allow countries that currently import natural gas to become self-sufficient. The current challenge is to inventory this resource and find safe, economical ways to develop it.
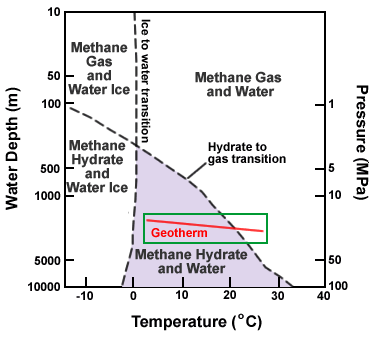
Methane hydrate stability chart: This phase diagram shows water depth (pressure) on the vertical axis and temperature on the horizontal axis. The dashed lines separate stability fields of water, water ice, gas and gas hydrate. The line labeled "hydrate to gas transition" is significant. Conditions for the formation of methane hydrate occur below this line. Above this line methane hydrate will not form. The red line traces a geotherm (the change of temperature with depth at a specific location). Note how, as depth increases, the geotherm crosses the hydrate to gas transition line. This means that gas hydrate in sediments usually overlies free gas. Graph modified after NOAA. [4]
What is Methane Hydrate?
Methane hydrate is a crystalline solid that consists of a methane molecule surrounded by a cage of interlocking water molecules (see image at the top of this page). Methane hydrate is an "ice" that only occurs naturally in subsurface deposits where temperature and pressure conditions are favorable for its formation. These conditions are illustrated in the phase diagram on this page.
If the ice is removed from this temperature/pressure environment, it becomes unstable. For this reason methane hydrate deposits are difficult to study. They cannot be drilled and cored for study like other subsurface materials because as they are brought to the surface, the pressure is reduced and the temperature rises. This causes the ice to melt and the methane to escape.
Several other names are commonly used for methane hydrate. These include: methane clathrate, hydromethane, methane ice, fire ice, natural gas hydrate, and gas hydrate. Most methane hydrate deposits also contain small amounts of other hydrocarbon hydrates. These include propane hydrate and ethane hydrate.
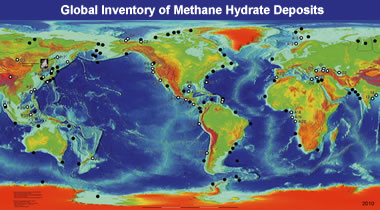
Methane hydrate map: This map is a generalized version of locations in the USGS global inventory of natural gas hydrate occurrence database. [2]
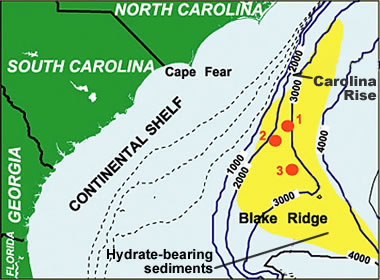
Gas hydrate map: One of the most extensively studied gas hydrate deposits is Blake Ridge, offshore North Carolina and South Carolina. Challenges of producing methane from this deposit are the high clay content and the low methane concentration. [3] This map is an example of the proximity of continental margin deposits to potential natural gas markets. Image by NOAA. [4]
USGS Gas Hydrates Lab: This video takes you on a visit to the USGS Gas Hydrates Lab where researchers conduct experiments on samples of gas hydrates collected from polar and continental margin areas. They also create synthetic gas hydrates and conduct experiments to determine their chemical and physical properties.
Where Are the Methane Hydrate Deposits?
Four Earth environments have the temperature and pressure conditions suitable for the formation and stability of methane hydrate. These are: 1) sediment and sedimentary rock units below Arctic permafrost; 2) sedimentary deposits along continental margins; 3) deep-water sediments of inland lakes and seas; and, 4) under Antarctic ice. [10]. With the exception of the Antarctic deposits, methane hydrate accumulations are not very deep below Earth's surface. In most situations the methane hydrate is within a few hundred meters of the sediment surface.
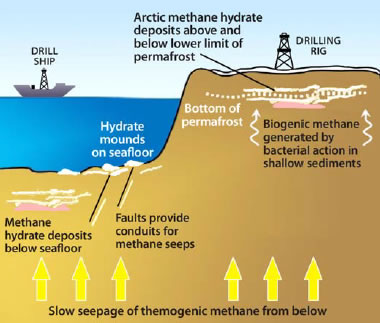
Methane hydrate deposit models: Deposit models for methane hydrate deposits at continental margins and under permafrost. [7]
In these environments methane hydrate occurs in the sediment as layers, nodules, and intergranular cements. The deposits are often so dense and laterally persistent that they create an impermeable layer that traps natural gas moving upwards from below.
In 2008, the United States Geological Survey estimated the total undiscovered gas hydrate resource for the Alaska North Slope area. They estimate that the total undiscovered natural gas resource in the form of gas hydrate ranges between 25.2 and 157.8 trillion cubic feet. Because very few wells have been drilled through the gas hydrate accumulations, the estimates have a very high level of uncertainty. [5]

Gas hydrate well: Ignik Sikumi #1 gas hydrate well on the Alaska North Slope. A USGS gas hydrate resource assessment determined that the North Slope has an extensive gas hydrate resource trapped below permafrost. Department of Energy photo.
Ignik Sikumi: This video takes you on a visit to the Ignik Sikumi gas hydrate field trial, a well on Alaska's North Slope that produced natural gas from gas hydrates below permafrost. The accomplishment made here was to liberate the methane by replacing it with carbon dioxide - without melting the gas hydrate.
Where is Methane Hydrate Produced Today?
To date there has been no large-scale commercial methane production from gas hydrate deposits. All of the production has either been small scale or experimental.
In early 2012, a joint project between the United States and Japan produced a steady flow of methane by injecting carbon dioxide into the methane hydrate accumulation. The carbon dioxide replaced the methane in the hydrate structure and liberated the methane to flow to the surface. This test was significant because it allowed the production of methane without the instabilities associated with a melting gas hydrate. [6]
The most likely methane hydrate deposits to be selected for first development will have the following characteristics: 1) high concentrations of hydrate; 2) reservoir rocks with high permeability; and, 3) locations where there is an existing infrastructure. [7] Deposits meeting these characteristics will likely be located on the Alaska North Slope or in northern Russia.
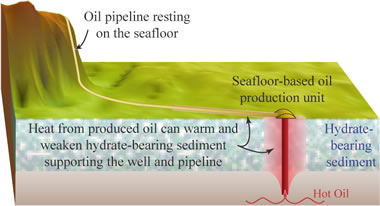
Gas hydrate melting: When oil wells are drilled through hydrate-bearing sediments, the warm temperature of the oil moving up through the frozen hydrate zone can cause melting. This can result in well failure. Warm pipelines running over frozen hydrate outcrops is also a hazard. [8] USGS image.
| Did You Know? Methane hydrate has a very high concentration of methane. If you melt a one-cubic-meter block of methane hydrate, about 160 cubic meters of gaseous methane will be released. |
Methane Hydrate Hazards
Methane hydrates are sensitive sediments. They can rapidly dissociate with an increase in temperature or a decrease in pressure. This dissociation produces free methane and water. The conversion of a solid sediment into liquids and gases will create a loss of support and shear strength. These can cause submarine slumping, landslides, or subsidence that can damage production equipment and pipelines. [7]
Methane is a powerful greenhouse gas. Warmer Arctic temperatures could result in gradual melting of gas hydrates below permafrost. Warming oceans could cause gradual melting of gas hydrates near the sediment-water interface. Although many news reports have presented this as a potential catastrophe, USGS research has determined that gas hydrates are currently contributing to total atmospheric methane and that a catastrophic melting of unstable hydrate deposits is unlikely to send large amounts of methane into the atmosphere. [9]
| Methane Hydrate References |
|
[1] USGS Gas Hydrates Lab: Stephen Wessells, Laura Stern, Steve Kirby; United States Geological Survey Multimedia Gallery Video, 2012.
[2] A Global Inventory of Natural Gas Hydrate Occurrence: Keith A. Kvenvolden and Thomas D. Lorenson, Pacific Coastal & Marine Science Center, United States Geological Survey. [3] Natural Gas Hydrates: A Review: Timothy S. Collett, Arthur H. Johnson, Camelia C. Knapp, Ray Boswell; Natural gas hydrates--Energy resource potential and associated geologic hazards: AAPG Memoir 89, p. 146-219, 2009. [4] Gas Hydrates Offshore Southeastern United States: Carolyn Ruppel, Georgia Institute of Technology, NOAA Ocean Explorer website, last accessed July 2022. [5] Assessment of Gas Hydrate Resources on the North Slope, Alaska, 2008: United States Geological Survey, Fact Sheet 2008-3073, October 2008. [6] U.S. and Japan Complete Successful Field Trial of Methane Hydrate Production Technologies: United States Department of Energy press release, May 2, 2012. [7] Energy Resource Potential of Methane Hydrate: An introduction to the science and energy potential of a unique resource; publication by the National Energy Technology Laboratory, United States Department of Energy, February 2011. Archived copy. [8] Pure phase thermal property results: sI Methane Hydrate: Woods Hole Science Center, United States Geological Survey, 2007. Archived copy. [9] Gas Hydrates and Climate Warming - Why a Methane Catastrophe Is Unlikely: Carolyn Ruppel and Diane Noserale, United States Geological Survey, Sound Waves Newsletter, May/June 2012. [10] Study suggests large methane reservoirs beneath Antarctic ice sheet: Tim Stephens, press release, University of California Santa Cruz, August 29, 2012. |
Enormous Potential
Although methane hydrate accumulations are located in difficult environments and present numerous technical challenges, they are widely distributed and the largest source of hydrocarbons on Earth. A variety of technologies could be developed to produce them using pressure reduction, ion exchange, and other processes that take advantage of their unique chemical and physical properties. The United States, Canada, Japan, and India all have vigorous research programs working to discover viable technologies for producing gas hydrates. Methane hydrate will likely play an important role in our future energy mix.
| More Oil |
 |
The Doorway to Hell |
 |
Oil Sands |
 |
Shale Gas Resources |
 |
Gifts That Rock |
 |
Horizontal Drilling |
 |
Oil and Gas Rights |
 |
Natural Gas Prices |
 |
Oil Shale |

Find Other Topics on Geology.com:

|

| ||

|

| ||

|

| ||

|

|
