Serpentine
A group of minerals used as architectural, ornamental, and gem materials. A source of asbestos.
Article by: Hobart M. King, PhD, RPG

Lizardite: This is a specimen of lizardite, a serpentine-group mineral. This specimen has a gemmy green color and a very smooth texture. This specimen is suitable for cutting into a few gemstones. This specimen is about four centimeters across. From Warren County, New York.
What is Serpentine?
Serpentine is not the name of a single mineral. Instead it is a name used for a large group of minerals that fit this generalized formula:
(X)2-3(Y)2O5(OH)4.
In this formula, X will be one of the following metals: magnesium, iron, nickel, aluminum, zinc, or manganese; and, Y will be silicon, aluminum, or iron. The appropriate generalized formula is therefore as follows:
(Mg,Fe,Ni, Mn,Zn)2-3(Si,Al,Fe)2O5(OH)4.
Chrysotile, antigorite, and lizardite are three of the primary serpentine minerals. There are many other serpentine minerals, most of which are rare.
Serpentine group minerals have similar physical properties and form by similar processes. They often occur as fine-grained admixtures and can be difficult to distinguish within a rock. Geologists usually call these materials "serpentine" rather than more specific names to simplify communication.

Ophiolite: Surface exposure of an Ordovician ophiolite in Gros Morne National Park, Newfoundland. Ophiolites are occurrences of oceanic plate and/or mantle rock exposed at the surface. They usually consist of serpentinite and associated rocks. (GNU Free Documentation License Image).
Serpentinites and Serpentine Formation
Serpentine minerals form where peridotite, dunite, and other ultramafic rocks undergo hydrothermal metamorphism. Ultramafic rocks are rare at Earth's surface but are abundant at the oceanic moho, the boundary between the base of the oceanic crust and the upper mantle.
They are metamorphosed at convergent plate boundaries where an oceanic plate is pushed down into the mantle. This is where they are subjected to hydrothermal metamorphism. The source of water for this process is seawater entrained in the rocks and sediments of the oceanic slab.
During hydrothermal metamorphism, olivine and pyroxene minerals are transformed into or are replaced by serpentine minerals. Some of the metamorphic rocks produced here are composed almost entirely of serpentine minerals. These serpentine-rich rocks are known as "serpentinites."
Extensive areas of Earth's surface are underlain by serpentinites. These areas occur near present or ancient convergent plate boundaries. They are locations where remnants of an oceanic plate is exposed at the surface. The remnant portion of the plate was either thrusted up onto land, accreted onto the edge of a land mass, or exposed by uplift and deep weathering.
These areas of exposed oceanic plate are known as ophiolites. They are often the source of valuable minerals that might include magnetite, chromite, chrysoprase, jade, and serpentine.
Physical Properties of Serpentine |
|
| Chemical Classification | Silicate |
| Color | Usually various shades of green, but can be yellow, black, white, and other colors. |
| Streak | White |
| Luster | Greasy or waxy |
| Diaphaneity | Translucent to opaque, rarely transparent |
| Cleavage | Poor to perfect |
| Mohs Hardness | Variable between 3 and 6 |
| Specific Gravity | 2.5 to 2.6 |
| Diagnostic Properties | Color, luster, fibrous habit, hardness, slippery feel |
| Chemical Composition | (Mg,Fe,Ni,Al,Zn,Mn)2-3(Si,Al,Fe)2O5(OH)4 |
| Crystal System | Most serpentine minerals are monoclinic. |
| Uses | A source of asbestos, architectural stone, ornamental stone, gem material. |
Physical Properties of Serpentine
The most obvious physical properties of serpentine are its green color, patterned appearance, and slippery feel. These remind the observer of a snake and that is where the name "serpentine" was derived.
Serpentine is also known for its translucent diaphaneity, waxy luster, ease of being cut into shapes, and its ability to accept a polish. These properties make it a popular gemstone, architectural material, and ornamental stone.
Last is serpentine's ability to resist the transfer of heat. That makes it a valuable insulator. Fibrous varieties of serpentine, such as chrysotile, have been used to make asbestos, which has many industrial uses. Its use today is limited because the fibers have been associated with respiratory disease.
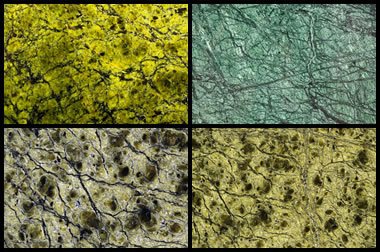
Architectural Serpentine: Serpentine has a long history of use as an architectural stone. It is usually green in color, cuts easily, polishes well, and has an attractive appearance. It was popular in the first half of the 20th century but is used less today, partly out of concern that it might contain asbestos. Enlarge image. Images copyright by iStockphoto and, clockwise from top left, Vladvg, Violetastock, AlexanderCher, and AlexanderCher.

The best way to learn about minerals is to study with a collection of small specimens that you can handle, examine, and observe their properties. Inexpensive mineral collections are available in the Geology.com Store. Image copyright iStockphoto / Anna Usova.
Use of Serpentine: Architectural Material
Serpentine has been used as an architectural stone for thousands of years. It is available in a wide variety of green and greenish colors, often has an attractive pattern, works easily, and polishes to a nice luster. It has a Mohs hardness of 3 to 6 which is softer than granite, and usually harder than most marble. This low hardness limits its appropriate use to surfaces that will not receive abrasion or wear, such as facing stone, wall tiles, mantles, and window sills.
Serpentine was popular in the United States during the first half of the 20th century and is less popular today. The decline in popularity is partly related to concerns about worker safety and the possible asbestos content of the stone.
In the dimension stone trade, serpentine is often sold as "marble." It might also be described as "serpentine marble" or given a trade name that does not include the word "serpentine." This is a tradition of the industry and is usually not a misidentification of the material. This practice severely irritates some geologists. :-)

Chrysotile: A rock containing chrysotile, a serpentine group mineral, with a fibrous habit in fractures. Specimen is approximately five centimeters across. From Easton, Pennsylvania.
Use of Serpentine: Asbestos
Some varieties of serpentine have a fibrous habit. These fibers resist the transfer of heat, do not burn, and serve as excellent insulators. The serpentine mineral chrysotile is common, found in many parts of the world, is easily mined, and can be processed to recover the heat-resistant fibers.
The use of chrysotile and other serpentine minerals with an asbestiform habit as insulators has been widespread. They were widely available, effective in their applications and inexpensive to produce. By the middle of the 20th century, they could be found in most buildings and vehicles. They were used to make wall and ceiling tiles, flooring, shingles, facing material, pipe insulation, stoves, paints, and many other common construction materials and appliances.
After they were discovered to be connected to lung and other cancers, their use was mostly discontinued, and a campaign to remove them from many of their uses began. Removal programs have been ongoing for decades and are still being done today. It has been one of the most costly removal programs in history.

Serpentine Cabochons: Three interesting cabochons cut from various types of serpentine. This is only a small example of the infinite diversity of serpentine gem materials.

Lime Green Serpentine: Rare specimens of serpentine have a wonderful green color, clarity, and translucence. These specimens have the appearance of nice jade and are sometimes confused with it in retail products.
Use of Serpentine: Gemstones
Attractive serpentine can be cut into a wide variety of gemstones. It is most often cut into cabochons and beads. They usually display a range of green, yellow, and black colors and often have magnetite, chromite, or other minerals as interesting inclusions. The lower left side of the green and black cabochon in the center of the photo on this page contains enough included magnetite that the cab can be moved with a small hand magnet.
Gemstone-quality serpentine is easy to polish, and beautiful finishes are possible. However, it usually polishes to a waxy luster rather than the brilliant glassy luster of much harder materials such as agate, jasper, and faceted stones. Rockhounds who polish their first piece of serpentine and know this have their expectations calibrated in advance. The waxy luster is a beautiful and common characteristic of the material. It does not reflect the skill of the operator. Extra polishing time and effort will still produce a waxy luster.
Serpentine has some durability concerns. It has a hardness that ranges from 3 to 6 on the Mohs scale. Three is far too soft for anything but the most gently-worn jewelry such as earrings, brooches, or pendants. A Mohs hardness of six is not hard enough for confident use in a ring or bracelet. Beads can be made from the more durable serpentine.
Some specimens of serpentine have a wonderful green color, clarity, and translucence. They are easily mistaken for fine jade by inexperienced buyers. The experienced buyer knows that serpentine polishes to a soft waxy luster rather than a bright glassy luster. Cabochons or beads with a waxy luster are not jade -- or they are jade with a poor polishing job.

Serpentine Sculpture: A small lion sculpture made of beautiful green serpentine by the House of Fabergé. This image by Shakko/Photos is displayed here under a Creative Commons License.
Use of Serpentine: Sculptures
Some varieties of serpentine can be carved into beautiful stone sculptures. Fine-grained, translucent material with a uniform texture and without voids and fractures is preferred. Serpentine is relatively soft and carves easily. It also accepts a nice polish.
Serpentine sculptures range in size from under one centimeter to several meters in height. Bowls, vases, desk sets, clock bases, animals, fruit, flowers, legendary figures, deities, busts, and statues are all common objects made by artists working with serpentine.
Use of Serpentine: CO2 Sequestration
Serpentinite rock units have been considered as repositories for the disposal of waste carbon dioxide produced when fossil fuels are burned. Injecting carbon dioxide into subsurface rock units in the presence of water can produce magnesium carbonate and quartz in an exothermic reaction similar to the one shown below.
Mg3Si2O5(OH)4 + 3CO2 + H2O --> 3MgCO3 + 2SiO2 + 3H2O
Numerous studies and small scale tests of geological sequestration of CO2 have produced promising results, but the procedure has not been placed into commercial practice.
Find Other Topics on Geology.com:

|

| ||

|

| ||

|

| ||

|

|









