Home » Rocks » Sedimentary Rocks » Limestone
Limestone
What is Limestone and How is it Used?
Article by: Hobart M. King, PhD, RPG

Limestone: A dark gray, fine-grained specimen of the Middle Mississippian Greenbrier Limestone from Randolph County, eastern West Virginia. Specimen shown is about two inches (five centimeters) across.
What is Limestone?
Limestone is a sedimentary rock composed primarily of calcite, a calcium carbonate mineral with a chemical composition of CaCO3. It usually forms in clear, calm, warm, shallow marine waters.
Limestone is usually a biological sedimentary rock, forming from the accumulation of shell, coral, algal, fecal, and other organic debris. It can also form by chemical sedimentary processes, such as the precipitation of calcium carbonate from lake or ocean water.
Table of Contents

A Limestone-Forming Environment: An underwater view of a coral reef system from the Kerama Islands in the East China Sea southwest of Okinawa. Here the entire seafloor is covered by a wide variety of corals which produce calcium carbonate skeletons. A United States Geological Survey image by Curt Storlazzi.
Biological Limestones
Most limestones form in calm, clear, warm, shallow marine waters. That type of environment is where organisms capable of forming calcium carbonate shells and skeletons can thrive and easily extract the needed ingredients from ocean water.
When these animals die, their shell and skeletal debris accumulate as a sediment that might be lithified into limestone. Their waste products also contribute to the sediment mass.
Limestones formed from this type of sediment are biological sedimentary rocks. Their biological origin is often, but not always, revealed in the rock by the presence of fossils.
Sometimes evidence of a biological origin is destroyed by the action of currents, organisms, dissolution, or recrystallization.
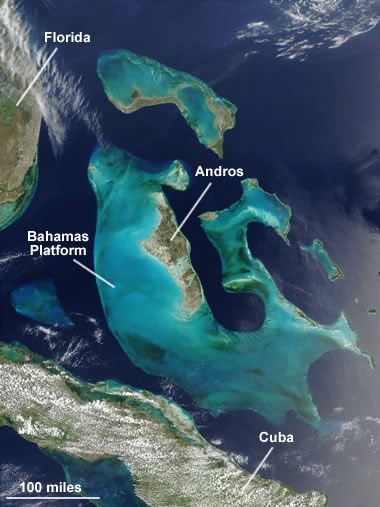
The Bahamas Platform: A NASA satellite image of the Bahamas Platform where active limestone formation occurs today. The main platform is over 100 miles wide, and a great thickness of calcium carbonate sediments have accumulated there. In this image the dark blue areas are deep ocean waters. The shallow Bahamas Platform appears as light blue. Enlarge image.
Chemical Limestones
Some limestones form by direct precipitation of calcium carbonate from marine or fresh water. Limestones formed this way are chemical sedimentary rocks. They are thought to be less abundant than biological limestones.
Most biological limestones contain significant amounts of directly precipitated calcium carbonate. After the biological grains have accumulated and are buried, water that is saturated with dissolved materials moves slowly through the sediment mass. Calcium carbonate, precipitated directly from solution, forms as a "cement" that binds the biological grains together.
"Cementation" is an important step in the transformation of a sediment into a rock. If the biological grains are not cemented together, a rock will not be formed. The amount of precipitated calcium carbonate in a biological limestone can be as low as a few percent of the rock by volume, or it can be higher than 50% of the rock by volume.
Limestone-Forming Environments
Many limestone-forming environments are active on Earth today. Most of them are found in shallow parts of the ocean between 30 degrees north latitude and 30 degrees south latitude.
Limestone is forming in the Caribbean Sea, Indian Ocean, Persian Gulf, Gulf of America, around Pacific Ocean islands, and within the Indonesian archipelago.
One of these areas is the Bahamas Platform, located in the Atlantic Ocean about 100 miles southeast of southern Florida (see satellite image). There, abundant corals, shellfish, algae, and other organisms produce vast amounts of calcium carbonate skeletal debris and fecal matter that completely blanket the platform. This is producing an extensive deposit of calcium carbonate sediment that has already converted to limestone at depth.
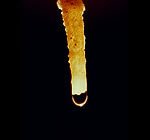 |
Limestone Stalactite A water drop clings to a stalactite. If it evaporates instead of falling, any dissolved calcium carbonate will add to the stalactite. National Park Service photo. |

Travertine: A view inside the Cave of Balzarca in the Czech Republic. Here stalactites, stalagmites, and flowstone adorn the roof, floor, and walls of the cave. These rocks are a variety of limestone known as travertine. Image copyright iStockphoto / JackF.
Evaporative (Cave) Limestones
Limestone can also form through evaporation. Stalactites, stalagmites, and other cave formations (often called "speleothems") are examples of limestone that formed through evaporation.
In a cave, droplets of water seeping down from above enter the cave through fractures or other pore spaces in the cave ceiling. There they might evaporate before falling to the cave floor.
When the water evaporates, any calcium carbonate that was dissolved in the water will be deposited. Over time, this evaporative process can result in an accumulation of icicle-shaped calcium carbonate on the cave ceiling. These features are known as stalactites.
If droplets fall to the floor and evaporate there, stalagmites could eventually grow upwards from the cave floor.
The limestone that makes up these cave formations is known as "travertine," a chemical sedimentary rock. A rock known as "tufa" is a limestone formed by evaporation at a hot spring or on the shoreline of a lake in an arid area.
Composition of Limestone
Limestone is by definition a rock that contains at least 50% calcium carbonate in the form of calcite by weight. All limestones contain at least a few percent other materials. These can be small particles of quartz, feldspar, or clay minerals delivered to the site by streams, currents and wave action. Particles of chert, pyrite, siderite, and other minerals can form in the limestone by chemical processes.
The calcium carbonate content of limestone gives it a property that is often used in rock identification - it effervesces in contact with a cold solution of 5% hydrochloric acid. See our article about the "acid test" for identifying carbonate rocks and minerals.
Types of Limestone
There are many different types of limestone - each with its own name. These names are often based upon how the rock formed, its appearance, its composition, or its physical properties. Here are some of the more commonly encountered types of limestone.

Chalk: A fine-grained, light-colored limestone formed from the calcium carbonate skeletal remains of microscopic marine organisms.
Chalk
Chalk is the name of a limestone that forms from an accumulation of calcareous shell remains of microscopic marine organisms such as foraminifera. It can also form from the calcareous remains of some marine algae.
Chalk is a friable limestone with a very fine texture, and it is easily crushed or crumbled. It is usually white or light gray in color.
In the past pieces of natural chalk were used to write on blackboards. Today, most blackboard chalk is a man-made product. Some of it is made from natural chalk along with additives that improve its performance.

Coquina: This photo shows the shell hash known as coquina. The rock shown here is about two inches (five centimeters) across.
Coquina
Coquina is the name of a poorly cemented limestone composed almost exclusively of sand-size fragments of calcareous shell and/or coral debris. A small amount of calcareous cement usually binds the grains together.
The sediments that form coquina accumulate on beaches where wave action delivers an abundance of locally produced biological grains, while a significant amount of other material is not deposited. Coquina might be composed of mollusk, gastropod, brachiopod, trilobite, coral, ostracod or other invertebrate remains. See accompanying photo or read an entire article about coquina here.

Crystalline Limestone: A specimen of limestone that has been subjected to metamorphism. Some might call this material "crystalline limestone" - however, the proper name is marble. If you view this rock closely by eye, or better, with a hand lens, you will clearly see cleavage faces of calcite intersecting at rhombic angles. The rock shown here is about four inches (ten centimeters) across.
Crystalline Limestone
When limestone is subjected to heat, pressure, and chemical activity, the calcite in the rock begins to transform. This is the beginning of the process known as metamorphism.
Starting at a microscopic scale, the calcium carbonate in the rock begins to crystallize or recrystallize into fine-grained calcite crystals. As the duration and intensity of metamorphism continues, the calcite crystals increase in size. When the calcite crystals are large enough to be visible to the eye, the rock can then be recognized as marble - a metamorphic rock.
Marble is the name of the metamorphic rock that forms when limestone is subjected to the heat and pressure of metamorphism. It is composed of calcium carbonate (CaCO3) and usually contains other minerals that might include clay minerals, micas, quartz, pyrite, iron oxide, and graphite.

Dolomitic Limestone: A view of the Kaibab Limestone at Walnut Canyon National Monument, Arizona. At this location, and many other locations, the Kaibab Limestone is fossiliferous and dolomitic. Photograph by the United States Geological Survey.
Dolomitic Limestone
Dolomitic limestone is a rock composed mainly of calcite, but some of that calcite has been altered to dolomite.
Dolomite is thought to form when the calcite (CaCO3) in carbonate sediments or in limestone is modified by magnesium-rich groundwater. The available magnesium facilitates the conversion of calcite into dolomite (CaMg(CO3)2). This chemical change is known as "dolomitization."
Dolomitization can completely alter a limestone into a dolomite, or it can partially alter the rock to form a "dolomitic limestone."

Fossiliferous Limestone: Ammonite fossils found in limestone quarry in Germany. Ammonite fossils are abundant in the area around Nuremberg and Stuttgart. Image copyright iStockphoto / hsvrs.
Fossiliferous Limestone
Fossiliferous limestone is a limestone that contains obvious and abundant fossils. They are usually marine invertebrates such as brachiopods, crinoids, mollusks, gastropods, and coral. These are the normal shell and skeletal fossils found in many types of limestone.
Fossiliferous limestone often contains information about the environment of deposition, and where the organisms lived (or were deposited). Paleontologists can often examine the fossils and determine the geologic age of the rock.

Lithographic Limestone: In 1908, workers at NOAA's printing shop ink a slab of lithographic limestone that contains an image of a nautical chart. In 1900, NOAA produced approximately 100,000 lithographic prints using this method. A crop from an image in the NOAA archive.
Lithographic Limestone
Lithographic limestone is a dense rock with a very fine and very uniform grain size. It occurs in thin beds which separate easily to form a very smooth surface.
In the late 1700s, a printing process known as lithography (named after the stones used) was developed to reproduce images by drawing them on the stone with an oil-based ink, then using that stone to press multiple copies of the image.
Lithographic printing developed into an art form that produced many of the finest maps, navigational charts, posters, and bookplates of the 18th and 19th century. It was used by NOAA and the United States military to produce millions of maps and navigational charts.
Printing with large stones weighing hundreds of pounds to over one ton was cumbersome work. Eventually lithographic printing was done using high-speed presses in which the image was inked on metal rollers and transferred onto sheets or rolls of paper as they streamed through the press.

Oolitic Limestone: A specimen of limestone composed almost entirely of oolites. This rock was collected from the Salem Limestone, Middle Mississippian, at an unrecorded / undisclosed site in southern Indiana. Photograph by James St. John, displayed here under a Creative Commons attribution license.
Oolitic Limestone
Oolites (or ooliths) are small, sand-size clasts of calcium carbonate with a spherical to ovate shape. They form by the concentric accumulation of calcium carbonate layers around a nucleus that might be a sand grain, a shell fragment, a coral fragment, or a particle of fecal debris. They are thought to form by inorganic precipitation of material around a nucleus while the clast is transported in wave-agitated waters or rolling across sediment surfaces.
In some parts of the Bahamas Platform, oolites are one of the most abundant clasts found in the sediment. In areas where currents from deep water ascend onto the platform, broad areas are covered by great thicknesses of sediment that is almost entirely oolitic.
Oolitic limestone is found in many parts of the world. Oolitic sediment is found in Great Salt Lake, Utah. Some sedimentary rocks are composed almost entirely of ooids and the calcium carbonate cement that binds them together.

Travertine used as flooring tile and wall panels in a modern home interior. Image copyright iStockphoto / Katarzyna Bialasiewicz.
Travertine
Travertine is a variety of limestone that forms where geothermally heated alkaline water, supercharged with dissolved gases and minerals, emerges at the surface. There, calcium carbonate and other minerals precipitate as the water degases and begins to evaporate.
Travertine can also form where these waters emerge into subsurface caverns. There, it can precipitate as cave formations such as stalactites, stalagmites, and flowstone.
When pure, travertine is white, but it is often stained by the presence of other minerals to cream, tan, greenish, brownish, and other colors. Because the precipitation is rapid and forms as encrustations on younger materials, travertine is often a banded rock with numerous voids and cavities. It sometimes contains inclusions of organic and mineral debris from the cave or surface environment.
Travertine was mined and used as an architectural stone in ancient Egypt and ancient Rome. Today, Egypt and Italy are famous sources of travertine that is exported throughout the world. It is sawn or sheared into floor tiles, window sills, wall panels, stair treads, and other shapes, mainly for interior use. High-quality material can sometimes accept a polish. The material can be recognized by its low hardness (3 on the Mohs scale), banded appearance, and porous texture.

Tufa is a porous rock that forms from the precipitation of calcium carbonate, often at a hot spring or along the shoreline of an alkaline lake where waters are saturated with calcium carbonate.

Tufa Towers are spectacular limestone features found in Mono Lake at Yosemite National Park in California. Image copyright iStockphoto / Andrew Soundarajan.
Tufa
Tufa is a porous limestone produced by precipitation of calcium carbonate from the waters of a hot spring or other body of surface water that has the ability to precipitate volumes of calcium carbonate. The pore space in tufa often results when plant material is trapped in precipitating calcium carbonate.
One of the most famous locations where tufa is actively forming is at Mono Lake, Yosemite National Park. The most spectacular tufa features at the lake are known as "tufa towers". They form by the interaction of freshwater springs and alkaline lake water.
Evaporation around the edges of the lake helps produce the jagged shoreline tufa deposits and a lake that is about 2 1/2 times as salty as the ocean and very alkaline.
In spite of its gnarly appearance as a rock, tufa actually has numerous architectural uses. When found in thick accumulations, tufa can be mined and sawn into blocks and sheets just like any other dimension stone. It produces a stone with a very rugged appearance.

Crushed Limestone: The Unsung Mineral Hero: Crushed stone is often looked upon as one of the lowliest of commodities; however, it is used for such a wide variety of purposes in so many industries that it should be elevated to a position of distinction. It is the geologic commodity upon which almost everything is built. The word cloud above shows just a few of its many diverse uses.
"Unsung Mineral Hero" is a quote from the late Dewey Kirstein, Economic Geologist and one of the author's early supervisors. [1]
Uses of Limestone
Limestone is a rock with a diversity of uses. It could be the one rock that is used in more ways than any other. Most limestone is made into crushed stone that is used in road base, railroad ballast, foundation stone, drainfields, concrete aggregate, and other construction uses. It is fired in a kiln with crushed shale to make cement.
Some varieties of limestone perform well in these uses because they are strong, dense rocks with few pore spaces. These properties enable them to stand up well to abrasion and freeze-thaw. Although limestone does not perform as well in these uses as some of the harder silicate rocks, it is much easier to mine and does not exert the same level of wear on mining equipment, crushers, screens, and the beds of the vehicles that transport it. In many parts of the world, the harder silicate rocks are too far from construction sites to be used economically.

A Gem of Crinoidal Limestone: This cabochon was cut from a piece of fossiliferous limestone that is rich in crinoid debris. Crinoids are organisms that have the morphology of stemmed plants but are actually animals. Rarely, crinoidal and other types of limestone have the ability to accept a bright polish and have interesting colors and patterns. These specimens can be made into unusual and beautiful organic gems. This cabochon is about 39 millimeters square and was cut from material found in China.

Anti-Skid Aggregate: This image is a microscopic view of a polished surface of the Loyalhanna Limestone from Fayette County, Pennsylvania. The Loyalhanna is a Late Mississippian calcareous sandstone to arenaceous limestone, composed of siliceous sand grains embedded in and bound by a matrix of calcium carbonate. In outcrop, the Loyalhanna is cross-bedded with features that have caused geologists to argue if it is of marine bar or eolian dune origin. This view shows about one centimeter of rock between opposing corners of the photo with sand grains measuring about 1/2 millimeter in diameter. As a construction material, the Loyalhanna is valued as an anti-skid aggregate (crushed stone). When it is used to make concrete paving, sand grains in aggregate particles exposed on a wet pavement surface provide traction for tires, giving the pavement an anti-skid quality.
Some additional but also important uses of limestone include:
Dimension Stone: Limestone is often cut into blocks and slabs of specific dimensions for use in construction and in architecture. It is used for facing stone, floor tiles, stair treads, window sills, and many other purposes.
Roofing Granules: Crushed to a fine particle size, crushed limestone is used as a weather- and heat-resistant coating on asphalt-impregnated shingles and roofing. It is also used as a top coat on built-up roofs.
Flux Stone: Crushed limestone is used in smelting and other metal refining processes. In the heat of smelting, limestone combines with impurities and can be removed from the process as slag.
Portland Cement: Limestone is heated in a kiln with shale, sand, and other materials and ground to a powder that will harden after being mixed with water.
AgLime: Calcium carbonate is one of the most cost-effective acid-neutralizing agents. When crushed to sand-size or smaller particles, limestone becomes an effective material for treating acidic soils. It has been widely used on fields and small plots throughout the world for hundreds of years.
Lime: If calcium carbonate (CaC03) is heated to high temperature in a kiln, the product will be a release of carbon dioxide gas (CO2) into the atmosphere and a residual product of calcium oxide (CaO). The calcium oxide is a powerful acid-neutralization agent. It is widely used as a soil treatment agent (faster acting than aglime) in agriculture and as an acid-neutralization agent by the chemical industry.
Animal Feed Filler: Chickens need calcium carbonate to produce strong eggshells, so calcium carbonate is often offered to them as a dietary supplement in the form of "chicken grits." It is also added to the feed of some dairy cattle who must replace large amounts of calcium lost when the animal is milked.
Mine Safety Dust: Also known as "rock dust." Pulverized limestone is a white powder that can be sprayed onto exposed coal surfaces in an underground mine. This bright white coating improves illumination and reduces the amount of coal dust that becomes suspended in the air of the mine. This improves the air for breathing, and it also reduces the explosion hazard produced by particles of flammable coal dust suspended in the air.
Limestone has many other uses. Powdered limestone is used as a filler in paper, paint, rubber, and plastics. Crushed limestone is used as a filter stone in on-site sewage disposal systems. Powdered limestone is also used as a sorbent (a substance that absorbs pollutants) at many coal-burning facilities.
Limestone is not found everywhere. It only occurs in areas underlain by sedimentary rocks. When limestone is needed in other areas, buyers sometimes pay five times the mine-site cost of the stone in delivery charges so that limestone can be used in their project or process.

Rock & Mineral Kits: Get a rock, mineral, or fossil kit to learn more about Earth materials. The best way to learn about rocks is to have specimens available for testing and examination.
| Limestone Information |
| [1] Limestone: West Virginia's Unsung Mineral Hero: Dewey Kirstein; an article in Mountain State Geology magazine, published by the West Virginia Geological and Economic Survey; pages 25-28, 1984. |
| More Rocks |
 |
Tumbled Stones |
 |
Fossils |
 |
Geodes |
 |
The Rock Used to Make Beer |
 |
Topo Maps |
 |
Difficult Rocks |
 |
Fluorescent Minerals |
 |
Sliding Rocks on Racetrack Playa |

Find Other Topics on Geology.com:

|

| ||

|

| ||

|

| ||

|

|

