Home » Minerals » Arsenopyrite
Arsenopyrite
Mineral Properties and Uses
Article by: Hobart M. King, PhD

Arsenopyrite and milky quartz on limestone from the Smith Vein, Carrock Mine, Caldbeck Fells, Cumberland, Cumbria, England. Specimen is approximately 8.8 x 6.2 x 4.8 centimeters in size. Specimen and photo by Arkenstone / www.iRocks.com.
What is Arsenopyrite?
Arsenopyrite is an iron arsenic sulfide with a chemical composition of FeAsS. It is the most abundant arsenic-bearing mineral and the primary ore of arsenic metal. It is associated with other sulfide minerals in organic-rich sedimentary rocks, metamorphic rocks, and igneous rocks in many parts of the world.
In addition to being found in deposits that are large enough to be minable, arsenopyrite is widely distributed. It is not an uncommon mineral. However, it usually occurs in such small amounts and in such small particle sizes that it is easily overlooked.
Table of Contents
Caution: A Toxic Material
Arsenopyrite contains arsenic, a toxic metal. Arsenic and arsenic compounds have been used as active ingredients in insecticides, herbicides, pesticides, and chemical weapons.
The dust of arsenopyrite can be harmful if inhaled, and the fumes produced by heating arsenopyrite are toxic. Avoid situations and activities where contact with dust and fumes can occur. Wash your hands if you must handle specimens that contain arsenopyrite - or better yet, wear gloves.
Physical Properties of Arsenopyrite |
|
| Chemical Classification | Sulfide |
| Color | Silvery-white to steel gray; however, it easily oxidizes to slightly iridescent colors of pink, brown, or copper. |
| Streak | Gray to black. |
| Luster | Metallic |
| Diaphaneity | Opaque |
| Cleavage | Poor |
| Mohs Hardness | 5.5 to 6 |
| Specific Gravity | 5.9 to 6.2 |
| Diagnostic Properties | High specific gravity. Its silvery color on freshly broken surfaces can be used to separate arsenopyrite from pyrite and marcasite. Crystals are sometimes striated or twinned. A slight odor of garlic might be noticed when arsenopyrite is crushed, broken or scraped across a streak plate. A garlic odor is also released when arsenopyrite is heated to a temperature that causes alteration. WARNING: The fumes produced by heating can be toxic! Dust produced when working with arsenopyrite can be toxic if inhaled. |
| Chemical Composition | Iron arsenic sulfide, FeAsS |
| Crystal System | Monoclinic |
| Uses | The primary ore of arsenic metal. Arsenic is toxic to many organisms, and that is its role in insecticides, herbicides, pesticides, chemical weapons, and other poisons. Arsenic metal is used to produce specialty alloys. Oxides of arsenic are used to make pigments. Modern organic compounds are displacing arsenic from many of its traditional uses. |
Geologic Occurrence
Much of the arsenopyrite that has been mined formed as a high-temperature mineral in hydrothermal veins. It is often mined, together with other metallic minerals, from veins that might contain gold, silver, lead, tungsten, or tin.
In these deposits arsenopyrite usually occurs in massive and granular form. It is often intergrown with other sulfide minerals such as chalcopyrite, galena, pyrrhotite, pyrite, and sphalerite; precious metals such as gold and silver; or other minerals such as scheelite, cassiterite, and quartz.
Arsenopyrite has also been mined from sulfide deposits formed by contact metamorphism. It is occasionally found in pegmatites. Well-formed crystals of arsenopyrite are most often found in pegmatites, marbles, and dolomites that have been altered by contact metamorphism.
Significant amounts of arsenopyrite have been produced from deposits in Bolivia, China, England, Germany, Greece, Japan, Mexico, Spain, and Sweden. In North America deposits are located in Ontario, Canada, and in South Dakota, New Jersey, and New Hampshire in the United States.
Arsenopyrite and Glaucodot
In rare deposits, cobalt substitutes for some of the iron in the arsenopyrite crystal structure. This produces a solid solution series between arsenopyrite (FeAsS) and glaucodot ((Co,Fe)AsS). Depending upon the amount of glaucodot present, these deposits can sometimes be mined more profitably by processing the ore to recover both cobalt and arsenic.

Arsenopyrite: Massive granular arsenopyrite from Gold Hill, Utah. Specimen is approximately 10 centimeters across.
Weathering of Arsenopyrite
Arsenopyrite is unstable in most environments of Earth’s surface. It easily alters from its silver-white or steel-gray color to yield a pink, bronze, or brown tarnish that is sometimes iridescent.
If a geologist strikes the mineral with a rock hammer to view a fresh surface, or does a streak test, an odor of garlic might be detected. This is a characteristic of many arsenic minerals and a clue that arsenopyrite might be present.
Arsenopyrite often oxidizes to form scorodite, a hydrated iron arsenate mineral with a chemical composition of FeAsO4.2H2O. Scorodite often weathers to limonite, an amorphous, hydrated iron oxide of variable composition. In areas where mining has exposed large amounts of sulfide ores, arsenopyrite can be a contributor to acid mine drainage problems.
Arsenopyrite as a Source of Gold
Arsenopyrite sometimes contains particles of gold that are so small that they cannot be detected with a hand lens. This "invisible gold" can sometimes be recovered in economic quantities by crushing the ore, concentrating the heavy fraction, and treating the heavy fraction with cyanide to dissolve the gold.
The "invisible gold" occurs in two forms: 1) tiny particles of elemental gold; and, 2) gold that is chemically bound within the arsenopyrite. Elemental gold exposed by crushing can be removed with the cyanide. The chemically bound gold is more difficult to remove without smelting.

The best way to learn about minerals is to study with a collection of small specimens that you can handle, examine, and observe their properties. Inexpensive mineral collections are available in the Geology.com Store. Image copyright iStockphoto / Anna Usova.
Arsenopyrite By Other Names
The name "arsenopyrite" is a contraction of "arsenical pyrites," an archaic name used for the mineral. "Mispickel" is another archaic name for arsenopyrite that was commonly used in Europe.
Uses of Arsenopyrite and Arsenic
Arsenopyrite is the primary ore of arsenic metal. The mineral contains approximately 46% arsenic by weight. Arsenic metal is used to produce a variety of alloys. It was historically used to harden lead in ammunition, but this use was nearly eliminated by the late 1900s.
Arsenic trioxide (As2O3) can be produced by smelting arsenopyrite. Arsenic trioxide is used to produce a variety of insecticides, herbicides, pesticides, and chemical weapons. Arsenic compounds are also used in medicines, as pigments in paints, to produce color in fireworks, and to color glass. Nontoxic compounds are displacing arsenic from many of its traditional uses.
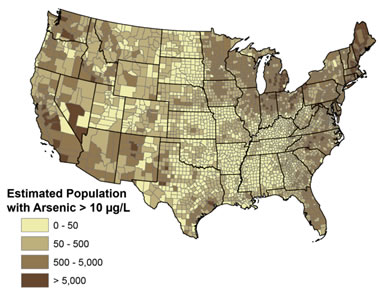
Arsenic in United States Groundwater: This map shows estimates of how many private domestic well users in each county may be drinking water with levels of arsenic of possible concern for human health (µg/L, micrograms per liter). Map from: Arsenic and Drinking Water, a report on the United States Geological Survey website, accessed October 2020.
Arsenic in Groundwater
In some parts of the world, groundwater contains enough dissolved arsenic to make it potentially harmful for human use. The two main ways that arsenic enters the groundwater are: 1) moving groundwater in contact with hazardous wastes, and, 2) moving groundwater dissolving arsenic from the rocks which it flows through. In the second case, the arsenic-bearing minerals are often arsenopyrite.
Arsenic in water has no smell and no taste, so a person might drink water containing arsenic without realizing it. The only way to determine if your well water contains arsenic is to have it tested and specifically ask the testing company to analyze for arsenic.
Health effects of dissolved arsenic in drinking water include:
 discoloration of the skin (see photos of this symptom in the hands of people suffering from arsenic poisoning in Bangladesh and a map of arsenic contamination in United States well water in this article) discoloration of the skin (see photos of this symptom in the hands of people suffering from arsenic poisoning in Bangladesh and a map of arsenic contamination in United States well water in this article) digestive problems that include: stomachache, nausea, vomiting, diarrhea digestive problems that include: stomachache, nausea, vomiting, diarrhea numbness in extremities numbness in extremities |
If you have a water well and want to learn more about arsenic in groundwater, see the Arsenic in Groundwater factsheet on the Illinois Department of Public Health website.
| More Minerals |
 |
Herkimer Diamonds |
 |
The Acid Test |
 |
Tumbled Stones |
 |
Zircon |
 |
Fool*s Gold |
 |
Kyanite |
 |
Rock Tumblers |
 |
Rhodochrosite |

Find Other Topics on Geology.com:

|

| ||

|

| ||

|

| ||

|

|
